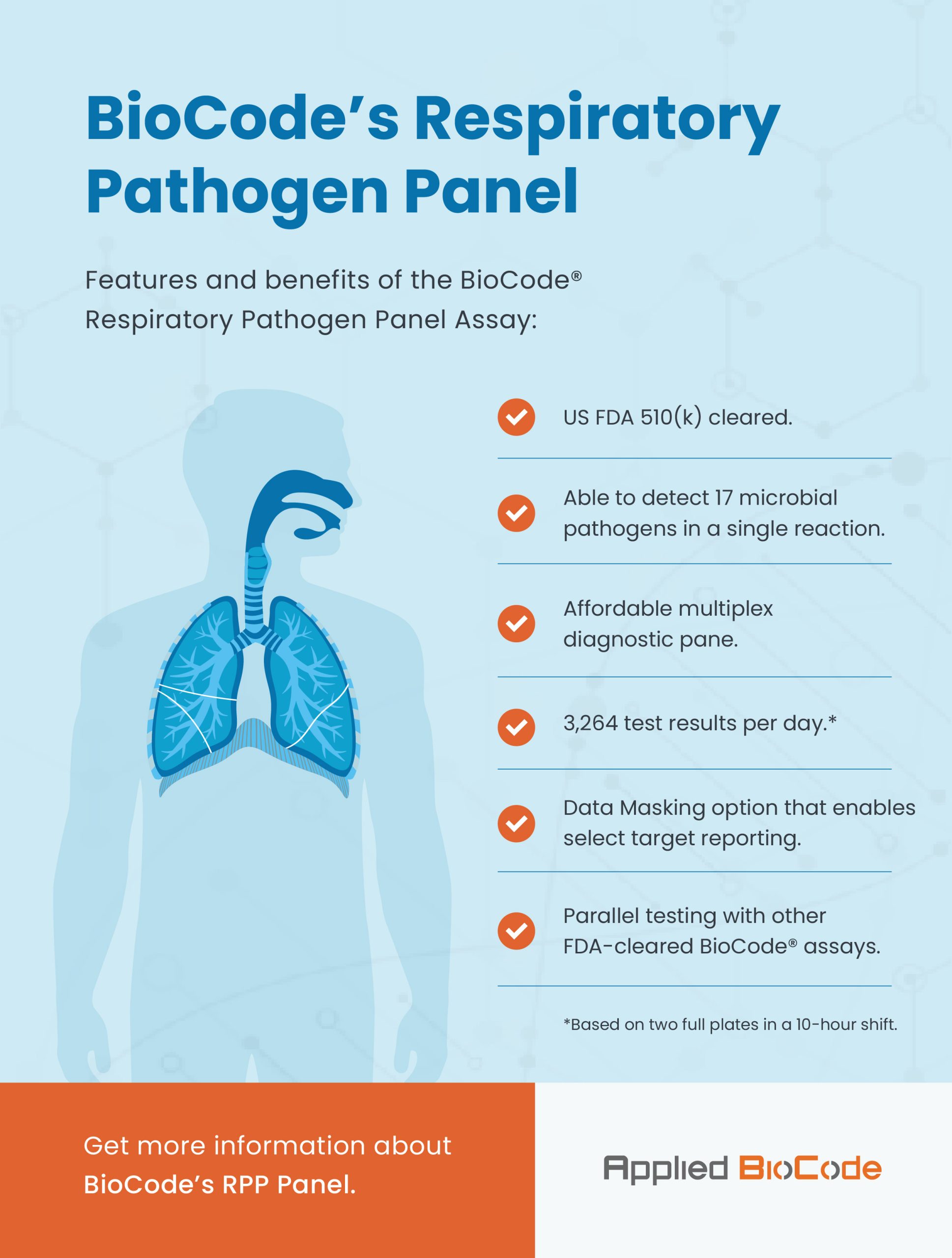
A respiratory panel that detects up to 17 pathogens in a single, all-in-one test.
The BioCode® Respiratory Pathogen Panel* (RPP) is a qualitative multiplexed nucleic acid-based in vitro diagnostic test that is capable of the simultaneous detection and identification of nucleic acids from multiple viruses and bacteria extracted from nasopharyngeal swab (NPS) samples obtained from individuals with signs and/or symptoms of respiratory tract infection.
* This product is for clinical and research applications, not individual use.