The BioCode® GPP Assay is an FDA 510(k) cleared, flexible, cost-efficient, and comprehensive molecular test.
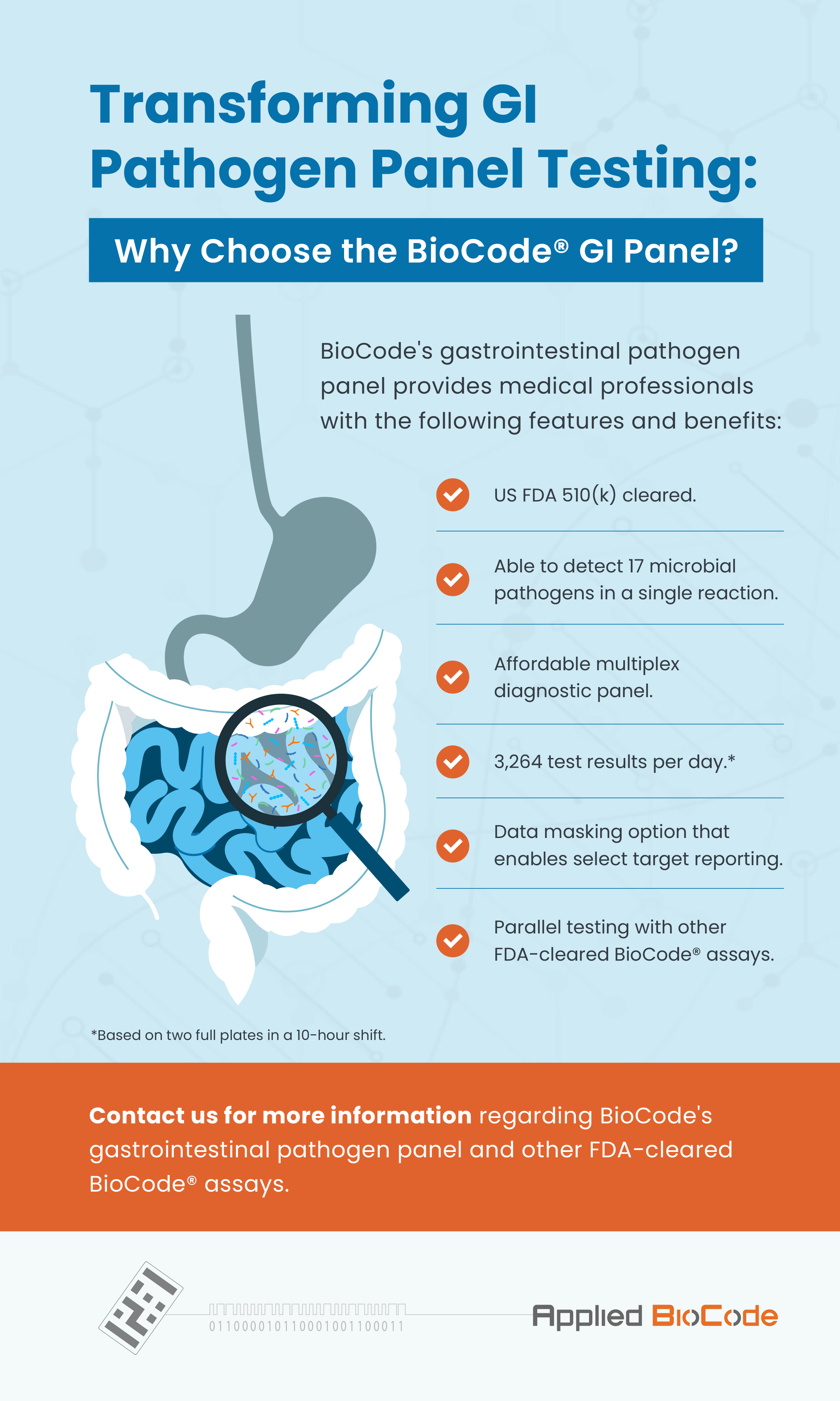
The BioCode® Gastrointestinal Pathogen Panel* utilizes proven Barcoded Magnetic Bead technology to detect specific gastrointestinal microbial nucleic acid from individuals exhibiting signs and symptoms of infections. This GI panel tests for 17 gastrointestinal pathogens in raw stool test specimens or stool in Cary-Blair medium from symptomatic patients.
* This product is for clinical and research applications, not individual use.